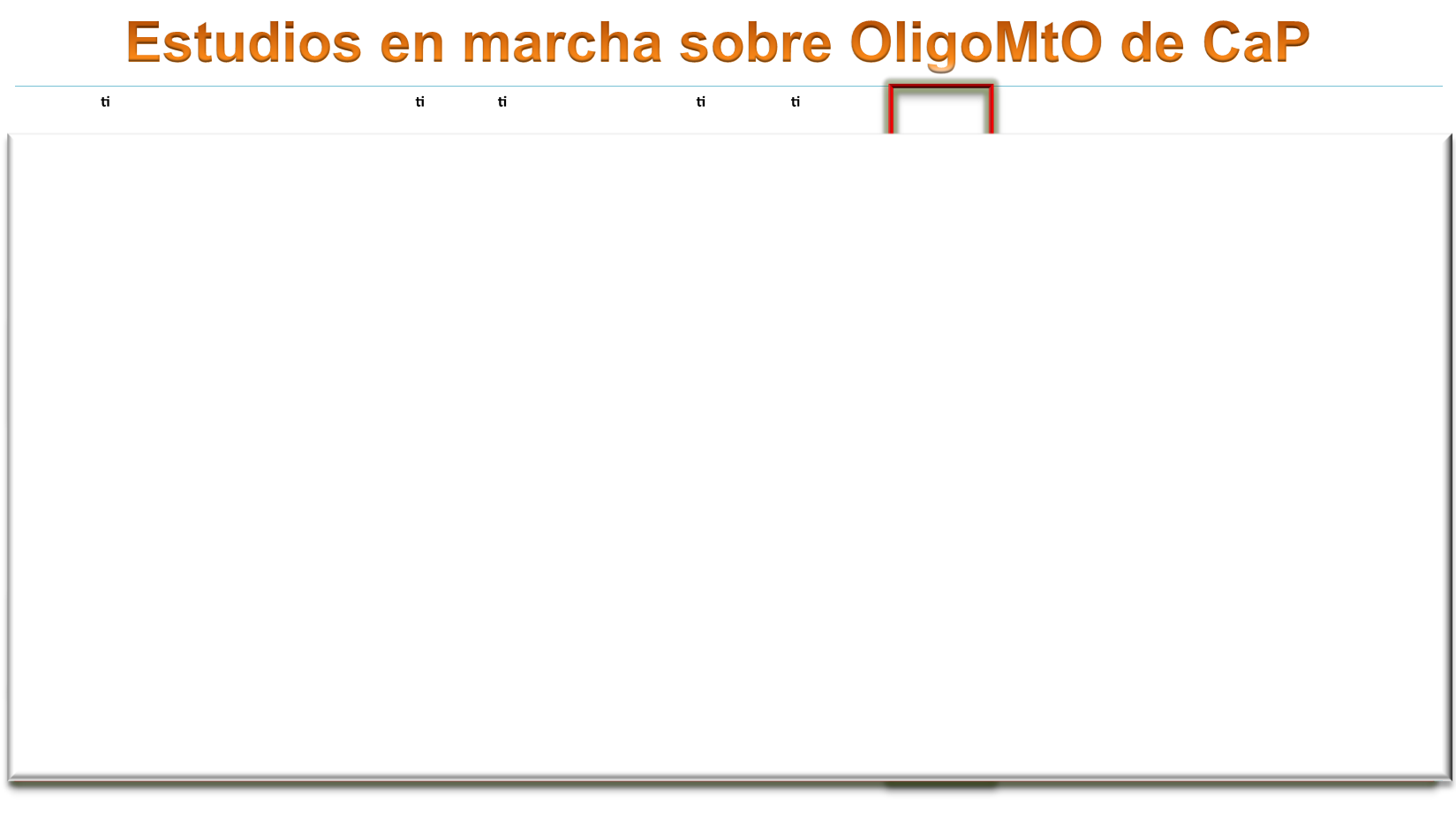
Study iden fier
Study type
Pa ent popula on
Popula on/ Interven on
N
Primary endpoint
Secondary endpoints
NCT01777802
Mayo Clinic
Observa onal
, prospec ve
Oligometasta c (4)
amen.to SABR
Monitor an -Pca immunity
following SABR
20
Induc on of
an prostate cancer
immunity
Not reported
NCT0256391
Toronto U
Phase I/II,
non
randdomized
Oligometasta c CS
(<5)
SABR, AD (intermi ent
HT allowed a er 1 y)
30
Rate of late
radiotherapy
toxicity
QOL, me to CR,LC,
Distant control, OS.
NCT01859221
University of
Florida
Phase II, non
randomized
CS or CRPC
oligometasta c
SABR/SHRT
48
PFS 78m
OS, treatment
failure rate; QOL
NCT01558427
Ghent U
Phase II,
randomized,
open label
Low volume
Oligorecurrent CS, ≤3,
Arm 1: ac ve
surveillance Arm 2:
(surgery or SBRT)
54
ADT-free survival
QOL
NCT02264379
Dresden U
Observa onal
, prospec ve
Oligomet. CS
oligorrec. (1–5)
Arm 1:5x5Gy
Arm2:3x10Gy
60
Toxicity (24 mo)
Acute toxicity, QOL,
LC, TFS, PSAFS
NCT02192788
SBRT-SG 05
GICOR
Phase II,
nonrandomized
oligorecurrent, <5
bone or lymph node
SABR
68
PFS (5 y)
OS; toxicity;TCT,
QOL.
NCT02685397
Jewish General
Hosp
Phase II/III
randomized
oligorecurrent,
<5 bone
or lymph node visceral
Arm 1:HT+Enza
Arm2:HT+Enza+SBRT
65
PFSr
QOL,Toxicity, LC,
TCT
TODAVIA NO SABEMOS CON ALTO NIVEL DE EVIDENCIA:
-Dosis máximas y seguras.
-Control Local y sintomático.
-Supervivencia Libre de Progresión.
-Si podemos retrasar o evitar la Hormonoterapia.
-O si hemos de asociar tratamiento sistémico y de qué tipo.


